Electron Microprobe Facility:
Earth's mantle and lower oceanic crust
- Ocean floor peridotites
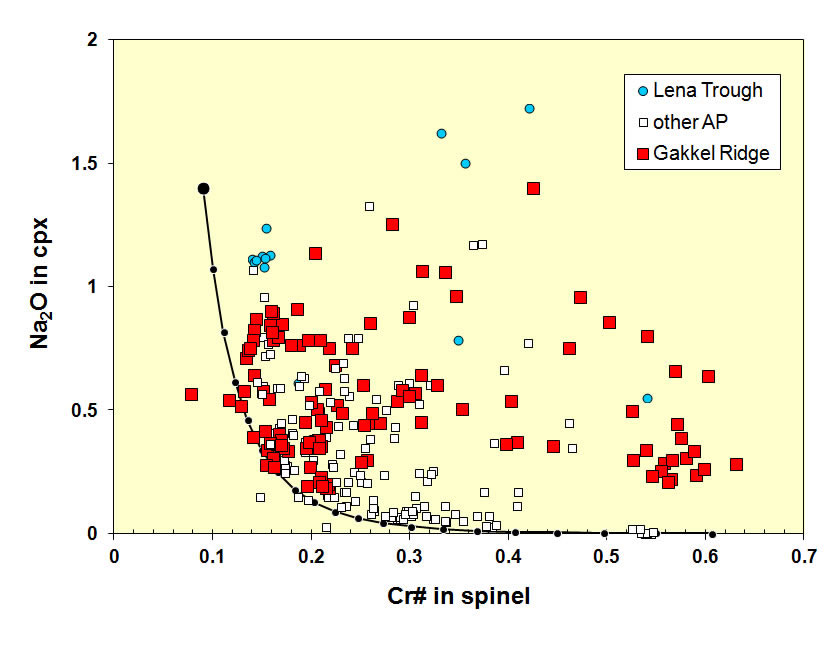
Ocean floor peridotites are to first order the residues of partial melting process that forms the oceanic crust. Their mineral proportions and compositions change systematically as fusible components are removed. Using the electron microprobe, we characterize the major element compositions of olivine, orthopyroxene (opx), clinopyroxene (cpx) and chromite. This provides a first-order estimate for the degree of depletion, and the necessary spatially controlled follow-up studies by SIMS, LA-ICP-MS, or other techniques.
The spinel Cr# (molar Cr/[Cr+Al]) is an indicator for the extent of depletion of a mantle peridotite. With increasing degrees of melting, Cr# increases, and Na, which preferentially enters the melt, is reduced in the coexisting cpx (black model curve). Many abyssal peridotites "have too much Na" at a given spinel Cr#, indicating that migrating melts can add Na back into the residue.
- Mantle xenoliths
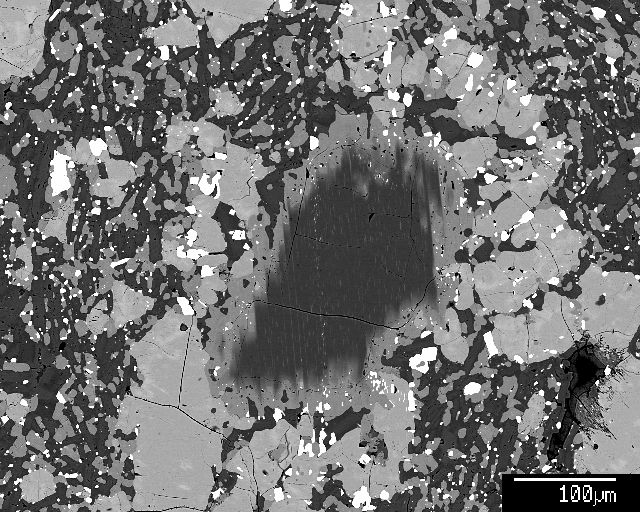
Many Hawaiian volcanoes have carried mantle xenoliths to the surface. They're most well known as fist-sized nodules, but also occur as slightly more inconspicuous single grains. Here are two examples of mantle opx, with typical fine cpx exsolution lamellae formed during slow cooling in the lithospheric mantle. The opx is strongly out of equilibrium with the melt that partially dissolved and reacted with the opx, forming a (protective) corona.
- Experiments: water in the deep mantle
Some of our experiments address the incorporation of water into nominally anhydrous mantle minerals under extremely high pressure. The two images below capture the phase transition of olivine (dark) to wadsleyite (bright), which occurs at the 410-km discontinuity. The high-pressure wadsleyite is more Fe- and water-rich than the equilibrium olivine.
More information on this fast reaction can be found in Smyth et al. (2012).
 |
 |
Other high-pressure experiments address the storage of water in so-called Dense Hydrous Magnesian Silicates (DHMS phases), such as Phase-D. An example of that is shown in the element map below.
More information on this study can be found in Hushur et al. (JGR, 2011).
-Si(Blue)_final.jpg)
X-ray distribution of Si (blue), and Mg (red) in high Si-activity sample, produced at lower mantle conditions (24 GPa). Stish (SiO2 stishovite), pv (MgSiO3 perovskite), D (phase-D), sB (superhydrous phase-B). Scale bar is 50 microns.
- Lower oceanic crust
Before erupt on the seafloor, Mid-Ocean Ridge Basalts undergo crystallization in lower crustal magma chambers. These magma chambers are normally not accessible, but some have been sampled during expeditions of the Ocean Drilling Program (ODP and IODP). We study the complexities of these lower crustal rocks to complement MORB studies. The rocks recovered range from very primitive olivine-rich troctolites to highly evolved zircon bearing granite-like rocks.

X-ray distribution map of the contact between an olivine-rich troctolite (left) and a gabbro (lower right). Olivine (green) has a cumulate texture, contains tiny chromites (red) and interstitial plagioclase (dark blue) and thin clinopyroxene films (light blue). The image is 2 cm wide. See Suhr et al. (2008).
Zircons from highly evolved lower crustal rocks contain age information. In collaboration with US and UK researchers, we studied mid-ocean ridge zircons from the Atlantic and Pacific Oceans and obtained ages of single zircon grains after detailed imaging and mapping. The images and yttrium maps were used on the cover page of a Nature Geoscience issue that accompanied the publication of an article by Rioux et al (2012).