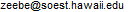 Professor
ProfessorDepartment of Oceanography
University of Hawaii at Manoa

Isotope Geochemistry
Stable isotope fractionation. Many powerful tools in biogeochemistry are based on stable isotopes. Isotopes are atoms of the same element but of different mass, for example 18O and 16O. The mass difference leads to small differences in the properties of isotopic molecules, which, for instance, causes small enrichments or depletions of the light or heavy isotope in different chemical compounds. This is called "isotope fractionation". The differences are usually of the order of per mil (‰; Greek symbol 'delta').
For example, there is a little bit more 18O than 16O in CaCO3 compared to the water from which the CaCO3 formed. The ratio is a function of temperature, which provides a means of taking Earth's temperature in the past (based on fossil CaCO3). In addition to temperature, also the growth and decay of the continental ice sheets during glacials/interglacials affect the oxygen isotope ratio preserved in carbonates (via the seawater ratio). 16O is preferentially deposited on the continents and removed from the ocean when sea level drops during glacials.
The oxygen isotope ratio of carbonates allows
reconstruction of temperature and continental ice volume changes, which have
varied periodically over the past 2 million years (data is available from
Lisiecki and Raymo). The pacemaker behind the major ice age terminations
is still somewhat of a mystery; see Schulz and Zeebe (2006) under
Publications.
Sediment Geochemistry. Sediments play an important role in the global biogeochemical cycling of the elements. For instance, deep-sea carbonate sediments are an essential component of the global carbon cycle. Projections of future fossil fuel neutralization and thus atmospheric CO2 levels depend on the sea-floor CaCO3 that is available to dissolution. Other examples include methane hydrate reservoirs in marine sediments, which have recently received strong attention because of their relevance to the carbon cycle and climate.
The term 'methane hydrate' describes a mixture of ice
and gas in which methane molecules are trapped within the crystal structure
of ice. They can form in deep-sea sediments under certain temperature and
pressure conditions and are often found at continental margins. Methane
hydrates have been referred to as 'burning ice'
(more).

In certain areas of the ocean, methane is released into the porewaters of
deep-sea sediments and the water column, where it can be utilized by microbes
and converted into CO2. For example, in areas such as the Hydrate
Ridge (Cascadia Margin, off Oregon), the porewater chemistry is dominated
by anaerobic oxidation of methane by microbial consortia.
In addition to its effect on the chemistry, methane
oxidation also dominates the stable carbon isotope composition of
dissolved inorganic carbon in porewater (delta-C-13).
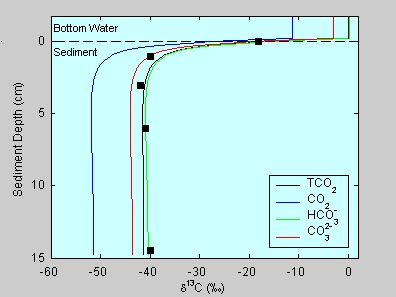
Biogenic methane
is isotopically 'light', that is, strongly depleted in C-13. When the methane
is oxidized to CO2, also the dissolved carbonate species become
isotopically 'light'. The figure shows model results (lines) for the carbon
isotope composition of porewater carbonate species and data
(d13C of TCO2, squares) at
Hydrate Ridge. Due to the effect of methane oxidation, porewater
carbonate species can be depleted by more than 50‰ relative to the bottom water;
see Zeebe (GCA, 2007) under
Publications.
Sediment-modeling studies complement our endeavors to decipher
isotope phenomena in biogenic CaCO3
(see Active Research Grants, National Science
Foundation: OCE05-25647).
Molecular Theory. The term isotope, which in Greek means equal places, reflects the fact that different atoms can occupy the same place in the Periodic Table. For example, hydrogen (1H) and deuterium (D = 2H) have nearly equal chemical properties, but D has twice the mass of H. For many other isotope systems, the mass difference is much smaller (e.g. only 13% for 18O and 16O).
Equilibrium isotope fractionation between chemical compounds is caused
by differences in the frequencies of the fundamental motions of isotopic
molecules - just as the oscillation frequency of two objects connected by
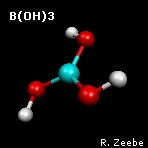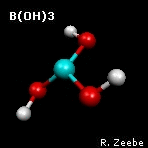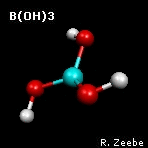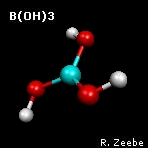
a spring depends on the masses of the objects. The animation shows the
"out-of-plane" mode of boric acid (calculations:
GAMESS
visualization:
Molekel). From the molecular
frequencies, isotope fractionation factors can be calculated that
are used in geochemistry; see Zeebe (2005) under
Publications for boron fractionation
factors.
Current projects focus on understanding and predicting isotope fractionation including elements such as H, B, C, O, P, S, and Ca.
Current projects focus on understanding and predicting isotope fractionation including elements such as H, B, C, O, P, S, and Ca.


Use browser-STOP/REFRESH buttons to stop/resume.

Department of Oceanography
University of Hawaii at Manoa
1000 Pope Road
Marine Sciences Building 629
Honolulu, HI 96822