Atmospheric Absorption of Energy
The sun primarily provides the energy that drives the Earth’s climate and weather by influencing atmospheric and surface processes (click here to see a related discussion of the Electromagnetic Spectrum - Box 1). The temperature of the surface of the sun is approximately 5480 oC and the radiation energy from the sun travels through space and impacts the Earth’s atmosphere. This radiation energy is primarily in the form of visible, short wave (ultraviolet), and long wave (infrared) radiation. Some of the sun’s energy is reflected back to space by the outer atmosphere, but the remainder of energy passes through the space-atmosphere boundary.
Figure 4. Illustration of relationship between radiation intensity and incoming solar radiation at the top of Earth’s atmosphere and outgoing Earth radiation. (Note: Much of the incoming shortwave UV solar radiation is absorbed by oxygen (O2 and O3) in the upper atmosphere. The outgoing long wavelength radiation emitted by the Earth is partially to totally absorbed by the greenhouse gases of water vapor (H2O), carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), and tropospheric ozone (O3).
The atoms and molecules that make up the atmosphere absorb the different types of radiation (visible, ultraviolet, and infrared) to varying degrees (see Figure 4). Oxygen, in the form of O2 (diatomic oxygen) and O3 (triatomic oxygen, ozone), is the most important absorber of incoming radiation in the atmosphere. High in the atmosphere, diatomic oxygen (O2) absorbs radiation with wavelength less than 240 nanometers (240 x 10-9 meters) and at lower altitude ozone (O3) absorbs radiation within the globally encircling stratospheric ozone layer with wavelengths mainly between 200 to 300 nanometers (200 to 300 x 10-9 meters). This incoming solar radiation is actually strong enough to break bonds holding the diatomic oxygen (O2) and ozone (O3) molecules together causing the molecule to split. Here are some equations representing those reactions.
O2 + solar radiation = O + O (1)
and
O3 + solar radiation = O2 + O (2).
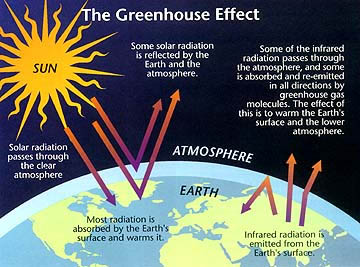
Most ozone in the atmosphere occurs in the stratosphere. The absorption of solar radiation by ozone in the stratosphere is the source for heat in the stratosphere and mesosphere (see Figure 3). The ozone also absorbs and blocks most ultraviolet radiation below 300 nanometers. As solar radiation continues to penetrate the atmosphere and gets closer to the Earth’s surface, it is scattered, reflected, and absorbed by air molecules, clouds, and various types of particles. Of the incoming solar radiation that hits the boundary between the Earth's atmosphere and outer space, about 30% is reflected back to space by atmospheric clouds and the Earth’s surface, 25% is absorbed by the atmosphere and reradiated back to space, and 45% is absorbed by the surface of land and ocean (Figure 5). The temperature of the Earth’s surface and lower atmosphere is higher than would be expected for a planet the distance of the Earth from the sun. This is because of the insulating qualities of the greenhouse gases in the Earth's atmosphere. When short wavelength radiation from the sun is not intercepted by the outer atmosphere or the ozone layer, it penetrates to the surface of the planet, is absorbed by the Earth's surface, and it is reradiated back as energy of a longer wavelength (infrared radiation) because the Earth is much cooler than the sun. Water vapor, carbon dioxide and other greenhouse gases absorb and trap this longer wavelength radiation leading to a natural warming of Earth’s surface and the lower atmosphere (see Figure 6). So it important to realize that the greenhouse gases don't trap incoming short wave radiation, but rather the long wave radiation that is emitted by the Earth's surface due to absorbing the short wave radiation.
Figure 5. Earth’s radiation budget. Incoming solar radiation is shortwave, ultraviolet, and visible radiation; outgoing Earth radiation is long wave infrared radiation.
The quantity of carbon dioxide residing in the atmosphere affects the amount of heat retained in the atmosphere and this in turn impacts climate. The more carbon dioxide (CO2) in the atmosphere, everything else being equal, the warmer the climate will be. Nitrous oxide (N2O), water vapor (H2O, the most important greenhouse gas), methane (CH4) and other gases have effects similar to those of carbon dioxide in controlling the amount of heat retained by the atmosphere. This overall process is the natural greenhouse effect. Without the naturally occuring greenhouse gases in the Earth’s atmosphere, the planetary surface temperature would be –18 oC. This is 33 oC cooler than its present average of 15 oC (see Box 2: Solar and Earth Radiation and the Greenhouse Effect). If there were no naturally occuring greenhouse gases, the Earth would likely be entirely covered in ice and so life as we know it would not exist.